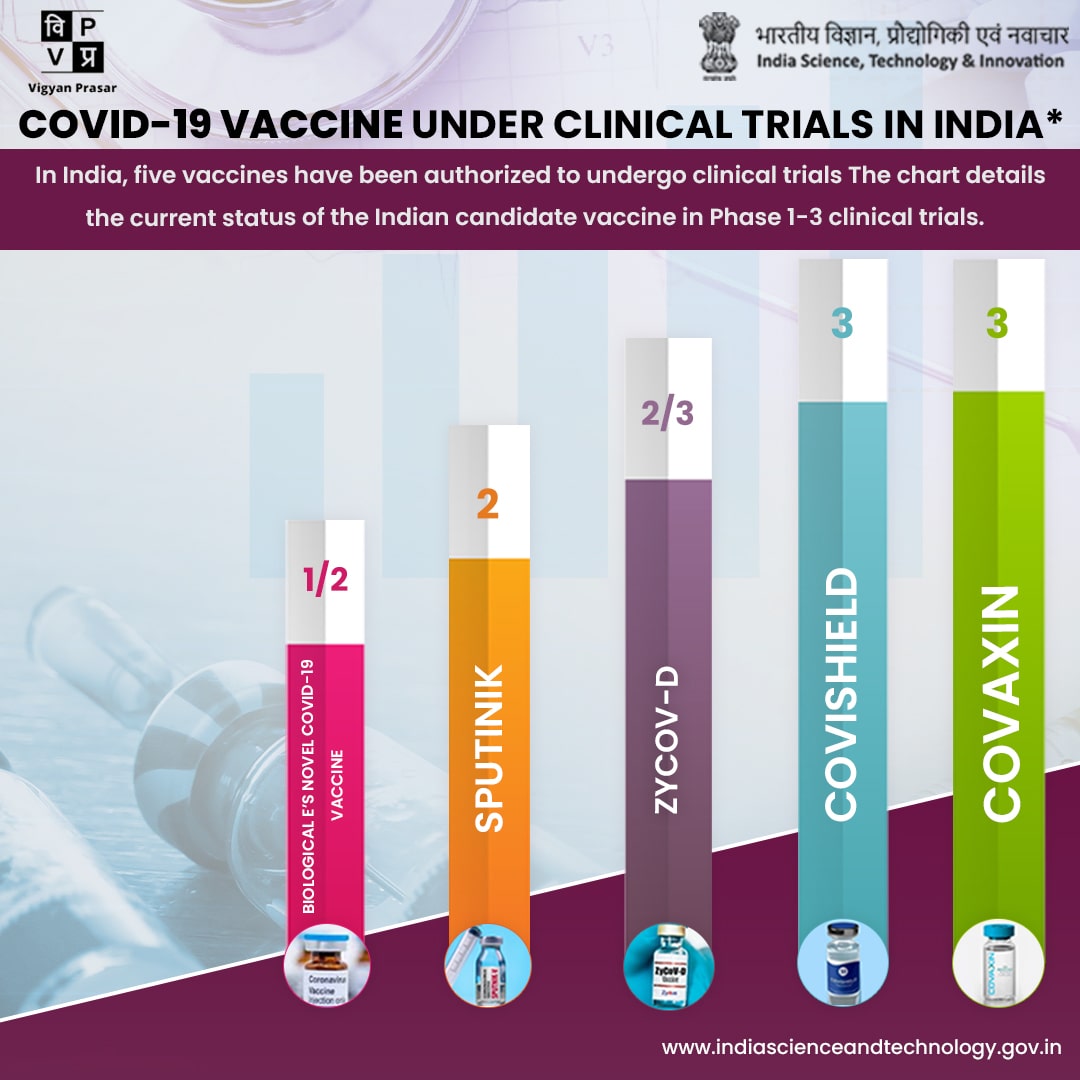
Globally there are more than 100 COVID-19 vaccine development projects underway, with several candidates already being tested on humans. Here is the list of vaccine which is of Indian origin and being tested on human:
COVAXIN: India's indigenous COVID-19 vaccine COVAXIN is developed by Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR) and National Institute of Virology (NIV). This indigenous, inactivated vaccine is developed and manufactured in Bharat Biotech's BSL-3 (Bio-Safety Level 3) high containment facility. The vaccine received approval from Drug Controller General of India (DCGI) for Phase I, II, III Human Clinical Trials. Phase I & II Human Clinical trials are completed and Phase III is in process.
Covishield
The Serum Institute of India (SII) and Indian Council of Medical Research are jointly conducting a Phase II/III, Observer-Blind, Randomized, Controlled Study to determine the safety and immunogenicity of Covishield (COVID-19 Vaccine). The vaccine received approval from Drug Controller General of India (DCGI) for Phase I, II, III Human Clinical Trials and they completed the Phase III Human Clinical Trials.
ZyCoV-D
Zydus Cadila, focused on discovering and developing NCEs, Novel Biologicals, Biosimilars and Vaccines, announced that its plasmid DNA vaccine to prevent COVID-19, ZyCoV-D. Safety in Phase I clinical trial of ZyCoV-D in healthy subjects established as endorsed by the independent Data Safety Monitoring Board (DSMB). Zydus commenced Phase II trial.
Sputnik
Dr. Reddy’s Laboratories Limited and Sputnik LLC are jointly conducting Multi-centre, phase II/III adaptive clinical trial to assess safety and immunogenicity of Gam-COVID-Vac combined vector vaccine.
The vaccine received approval from Drug Controller General of India (DCGI) for Phase I, II, III Human Clinical Trials and they are in process of Phase II clinical trial.
Biological E’s novel COVID-19 vaccine
Biological E. Limited is conducting a prospective open-label randomised Phase-I seamlessly followed by Phase-II study to assess the safety, reactogenicity and immunogenicity of Biological E’s novel COVID-19 vaccine containing Receptor Binding Domain of SARS-CoV-2 for protection against COVID-19 when administered intramuscularly in a two-dose schedule to healthy volunteers.
HGCO19 mRNA vaccine
Gennova, Pune supported with seed grant under the Ind-CEPI mission of Department of Biotechnology of M/o Science & Technology has received approval from Indian drug regulators to initiate Phase I/II human clinical trial.